
CLIA in medical billing is an amendment that Congress enacted in 1988 for labs working in the US. The three regulation bodies responsible for delivering CLIA certificates are the FDA, CMS and CDC. As a laboratory owner or worker, you must go through the application process to get CLIA certification. Need an in-depth overview of CLIA? This guide explores CLIA certification, its types, and how to get a CLIA number for your lab.
The CLIA full form in medical billing is Clinical Laboratory Improvement Amendments, an important certificate for day-to-day lab testing operations. It helps screen out patient health, detect and prevent patient disease, and assists clinicians in getting quick and accurate results.
These certificates are required for any level of laboratory operating in the USA. Lab operators must update their laboratory CLIA to the payer to get healthcare reimbursement without denial.
The purpose of CLIA in medical billing is to establish quality standards for all testing laboratories and ensure that patient test results are accurate and reliable.
This is crucial to delivering proper diagnoses and treatment decisions to patients. Without the CLIA certification, test accuracy could be compromised, and patients may receive inaccurate results, leading to incorrect medicine intake or serious health concerns.
A CLIA certificate is a federal requirement for laboratories to perform human-based testing, while a CLIA number is a 10-digit alphanumeric identifier assigned to that certificate. Below is the detailed breakdown of both terms.
CLIA certification is a formal authorization given to a laboratory before it starts performing lab testing on humans. If the laboratory performs tests outside its scope or is involved in a public health risk, CMS could suspend the laboratory, instantly ceasing all testing functions.
The CLIA number is a 10-digit alpha-numeric code provided to the laboratory when CMS certifies it. It identifies and verifies the CLIA certification for billing and regulatory purposes. When billing a claim to the payer, CLIA number in cms 1500 entered at box 33.
When applying for a CLIA in medical billing, ensure you know the CLIA certification types, which primarily focus on the laboratory’s complexity level and your service. Here’s a detailed understanding of each type.
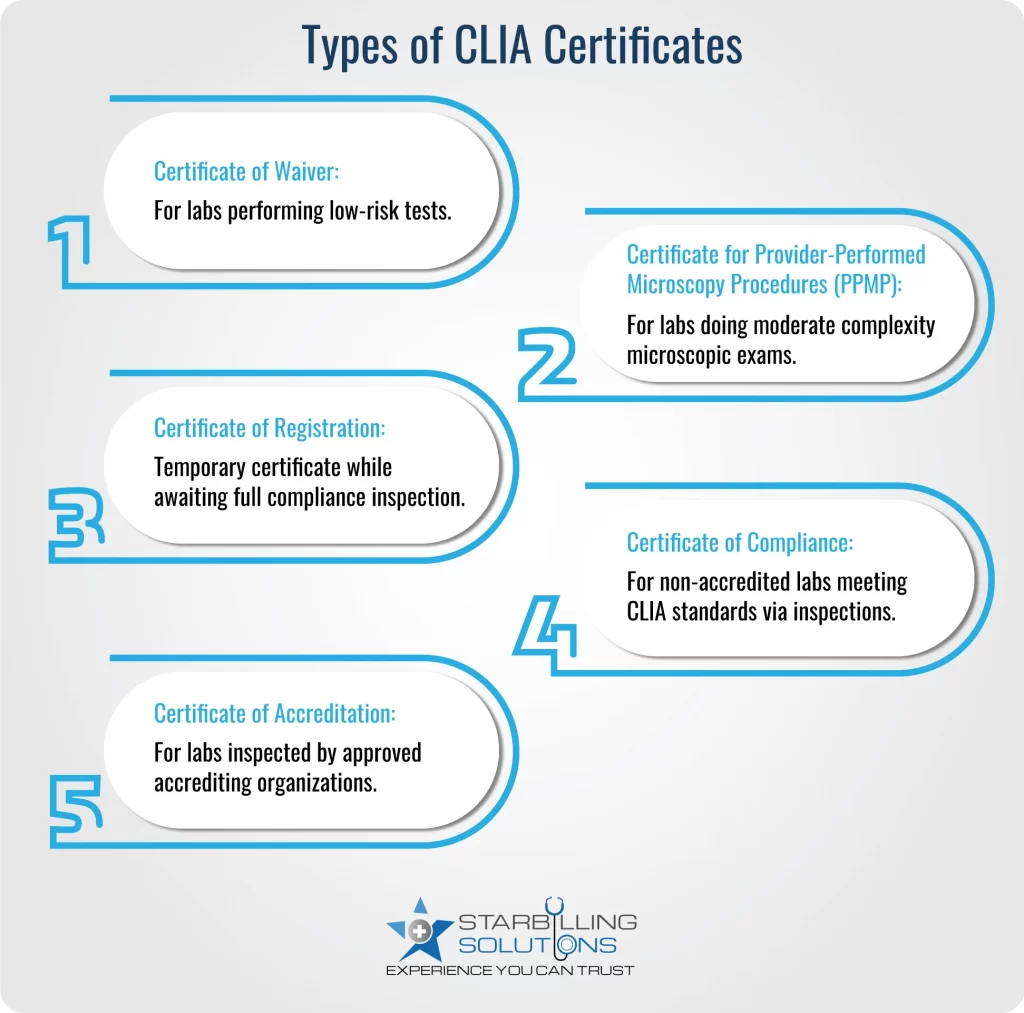
COW certification is issued to Labs that perform only CLIA waived tests (simple, low-risk tests approved by the FDA). These tests include blood glucose testing, urine pregnancy testing, and dipstick urinalysis. Acquiring COW is most suitable for physician offices, clinics and small facilities doing point-of-care testing.
This clinical laboratory improvement amendments certificate test is aligned with laboratories where physicians, mid-level practitioners, or dentists perform microscopy exams during patient visits. These testing laboratories are also eligible to perform waiver tests. Standard tests with the PPMP certificate include Wet mounts, KOH preps, semen analysis (post-vasectomy), and urine sediment exams.
A CLIA certificate of registration is a temporary certificate issued to laboratories that have applied for either a Certificate of Compliance or Accreditation. It is valid until the lab has been inspected and found compliant or accredited. Under this CLIA laboratory certificate, labs are eligible to conduct moderate or high-complexity testing while awaiting inspections.
A certificate of compliance CLIA is issued to the laboratory that performs high-end, complex testing. CMS issues this certificate after an onsite inspection of the lab. Certificates of this type must comply with all applicable CLIA regulations and be subject to routine inspections. Labs working independent or under hospitals, or speciality testing labs not under an accrediting organization, are most suitable for COC certification.
This certificate is issued to high-complexity testing labs that choose to be inspected by a CMS-approved accrediting organization, such as CAP, COLA, The Joint Commission, etc. To obtain a COA CLIA in medical billing, labs must meet CLIA and accreditor standards, which may be more stringent.
If your laboratory plans to test human specimens in the U.S., you must obtain a CLIA certificate. To get the CLIA laboratory certificate, the process includes filling out the form, understanding the credentials, and knowing where to submit it.
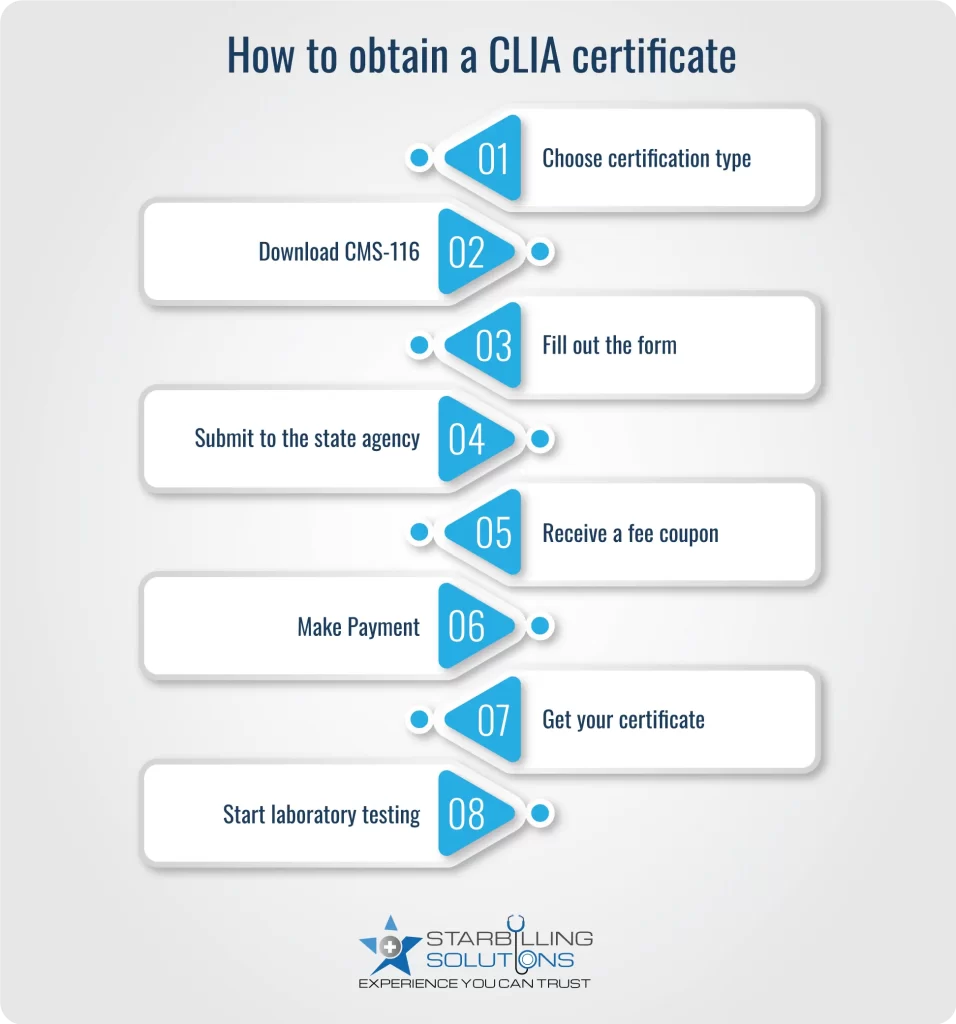
Note: Certificate of Waiver usually takes 30 days. While lab certificates with Moderate/High Complexity testing can take longer due to inspections and reviews
Every certified lab gets a CLIA number, a 10-digit alphanumeric identifier, which must be added to the claim form when billing for lab services. Here’s how, when, and where to include this in the claims form.
When: Whenever you bill a lab test performed in-house or by a reference lab under CLIA regulations.
Where: Enter the CLIA number in cms 1500 under box 23 for paper claim. For Electronic Claims (837P) use Loop 2300, segment REF01 = X4, REF02 = CLIA number.
How: The CLIA number must be active and match the NPI number. Make sure to use the correct CLIA certificate type.
Starting a medical laboratory is not easy, especially when getting CLIA certification or billing for a lab test. With the help of our expert billers, you could stay safe from both conditions. We ensure you get CMS approval and quick reimbursement when billing for a lab service. Our expert follows steps for CLIA in medical billing that include.
At Start Billing Solution, we offer expert CLIA certification and compliance assistance, so you won’t have to worry about slowing down your revenue cycle.
In medical billing, a CLIA ID is a 10-digit alphanumeric number used in the CMS 1500 claim form. It helps providers request reimbursements based on the lab services delivered.
The QW modifier is used for CLIA, which is appended to lab test CPT codes to indicate that the test is CLIA-waived. This means the test is simple and low-risk and doesn’t need a CLIA certificate when applying for a claim for reimbursement.
As of January 27, 2024, the fee for the CLIA certificate for different types is as follows.
There are 5 types of CLIA claims, each valid for 2 years.
New York and Washington are two states that are exempt from applying for a CLIA certificate. If your lab is in these states, contact their state office regarding a CLIA certificate.
Whatever your CLIA certificate type, if you make any changes in your laboratory, consult the state agency within 30 days. Some common changes include ownership, Name, Location, Laboratory Director, etc.
No, you can’t use another lab’s CLIA number. Every laboratory is assigned an individual number required for testing and billing purposes.